22 Embryology of the Gastrointestinal System
EMBRYOLOGY OF THE GASTROINTESTINAL SYSTEM
Learning Objectives
By the end of the course students will be able to:
1 . Describe the basic formation of the foregut, midgut and hind gut and how these components are distinguished on the basis of their blood supply
2. Describe the partitioning of the esophagus and trachea and the clinical consequences of their malformation
3.Understand how rotation of the stomach gives rise to the lesser and greater peritoneal sacs
4.Describe how the pancreas and biliary system develop from buds of the duodenum
5.Relate the process of “physiological herniation” of the midgut.and abnormalities of rotation to clinical problems
6. Describe how some portions of the GI system become “secondarily peritoneal”
Reference: Larsen, W.J. Human Embryology 3rd Edition, chapter 9
We also recommend that you look at the excellent tutorial on Gastrointestinal Embryology developed by the University of Indiana at:
http://www.indiana.edu/~anat550/gianim/index.html
and by the University of North Carolina at:
http://www.med.unc.edu/embryo_images/unit-digest/digest_htms/digesttoc.htm
Embryology of the Gastrointestinal System
Remember that: The embryonic GI tube may be divided into 3 parts:
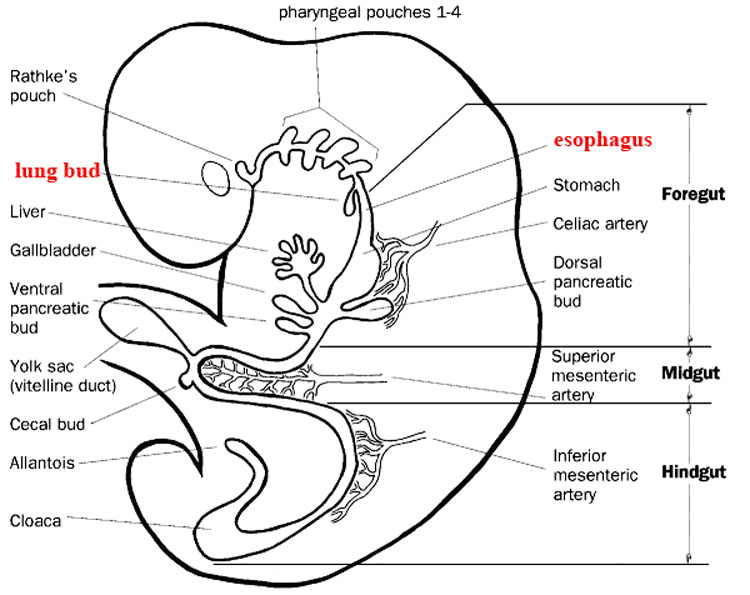
●Foregut, which begins at the level of the respiratory bud from the esophagus, and includes:
esophagus, stomach, duodenum to the level of the entry of the common bile duct (hepatopancreatic ampulla), liver and pancreas
●Midgut, which extends from the entry of the common bile duct to the junction of the proximal two-thirds and distal one-third of the transverse colon and includes:
distal duodenum, jejunum, ileum, cecum and vermiform appendix, ascending colon, and proximal transverse colon
●Hindgut, which extends from the junction of the proximal two-thirds/distal one-third of the transverse colon through the upper one-third of the rectum, and includes:
distal transverse colon, descending colon, sigmoid colon and upper one-third of the rectum/anal canal
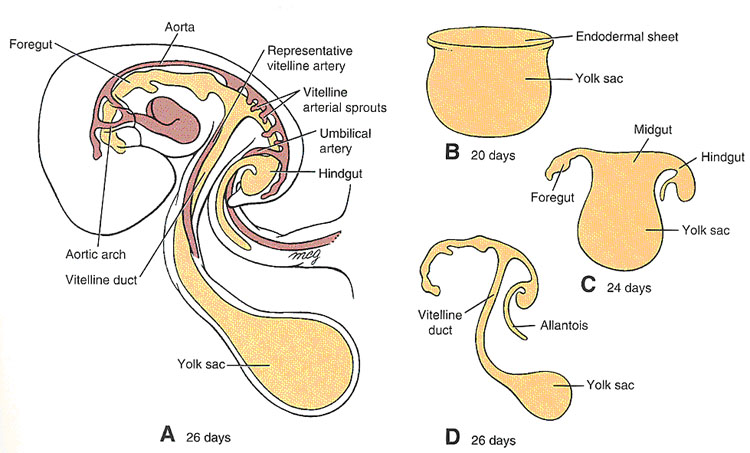
The derivatives of each of these divisions receive their arterial supply from branches of the original unpaired, visceral branches of the abdominal aorta that are derived from the vitelline arteries of the yolk sac:
● Celiac trunk: artery of the foregut
● Superior Mesenteric Artery: artery of the midgut
● Inferior mesenteric Artery: artery of the hidgut
Early in development, the embryo undergoes cephalocaudal (longitudinal) and lateral folding (Larsen, Fig 9-1) which result in incorporation of the part of the yolk sac destined to become the gut, and formation of the intraembryonic coelom, which will give rise to the pleural and pericardial cavities in the thorax, and the peritoneal cavity below the diaphragm. These body cavities, the true “inside” of the body, are lined by a specialized simple squamous epithelium known as mesothelium. This epithelium secretes a watery fluid: hence it and its underlying thin layer of loose connective tissue are known as serous membranes. In the abdomen, the portion of the serous membrane which lines the body wall is called the parietal peritoneum; the portion which invests many of the abdominal organs (viscera) is the visceral peritoneum. Organs suspended in the peritoneal cavity by double-layered folds of peritoneum, known as mesenteries, are considered to be “intraperitoneal”; structures which do not have mesenteries, and reside behind the posterior layer of the parietal peritoneum, are called retroperitoneal.
In addition to suspending viscera in the peritoneal cavity, the double-layered mesenteries have another function; they provide pathways for blood vessels, nerves and lymphatics to reach the viscera.
The foregut mesenteries are sites of organ formation (liver, gall bladder, pancreas, spleen) and give rise to peritoneal ligaments and omenta. Originally, there exist both a dorsal mesentery (dorsal mesogastrium) and ventral mesentery (ventral mesogastrium) in the foregut region. Midgut and hindhut are suspended only by dorsal mesentery throughout their development. Liver, gall bladder, and the ventral bud of the pancreas all develop within the ventral mesentery. Adult derivatives of the ventral mesentery include the lesser omentum and falciform ligament.
Development of Foregut
Partitioning of esophagus and trachea
The lower respiratory system (from the pharynx down)
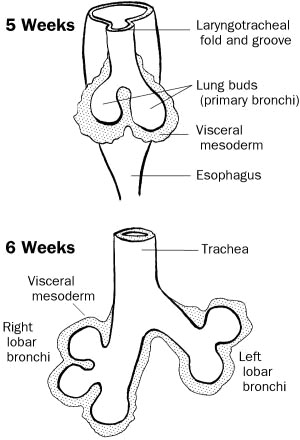
- develops during week 4 (26-27 days)
- starts as a median laryngotracheal groove in the caudoventral wall of the primitive pharynx.
- The endoderm lining the groove gives rise to the epithelium and glands of the larynx, trachea, bronchi and the pulmonary epithelium.
- Connective tissue, cartilage and smooth muscle of these structures develop from the splanchnic mesenchyme surrounding the foregut.
|
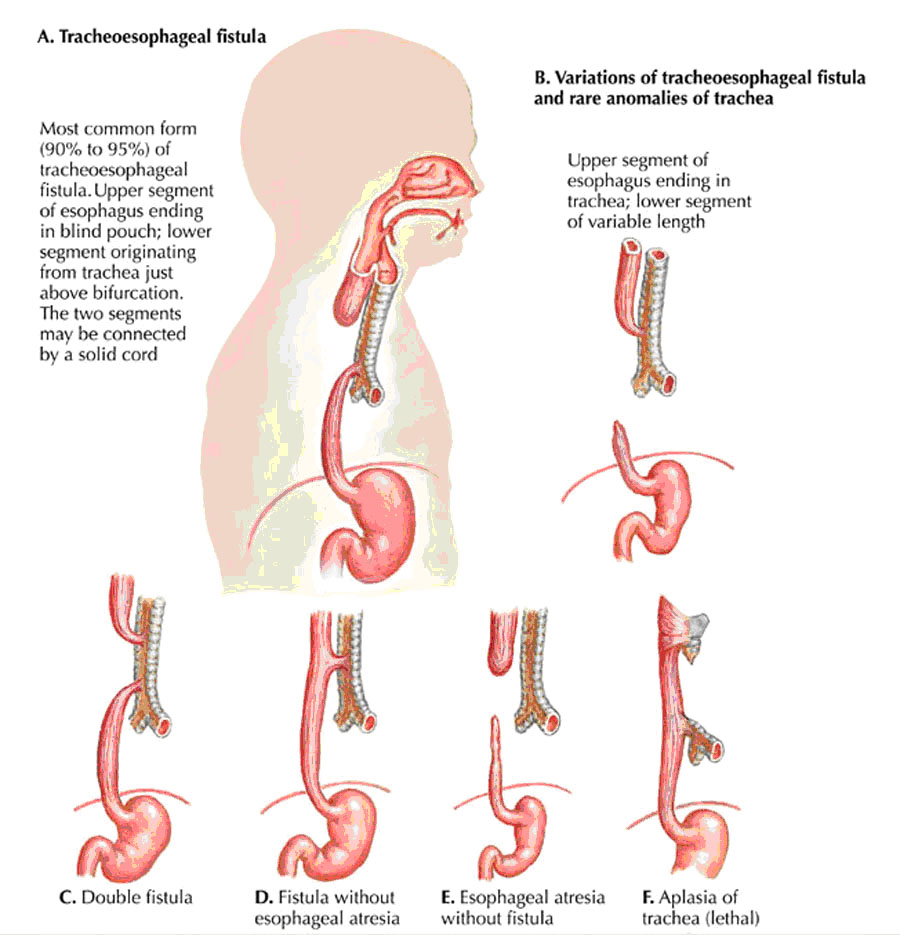
The laryngotracheal groove deepens into a diverticulum ventrally which enlarges distally into a lung bud The diverticulum becomes separated from the primitive pharynx by longitudinal trachoesophageal folds which fuse to form the trachoesophageal septum, dividing the foregut into the ventral laryngotracheal tube and the dorsal esophagus. |
|
Clinical Correlation: A fistula may exist connecting trachea and esophagus and resulting in abnormal communication between the 2.
|
The lung bud develops into 2 endodermal bronchial buds which grow into the pericardioperitoneal cavities, the primordia of the pleural cavities.
- Early in week 5, each bronchial bud enlarges into the primordium of a primary bronchus. The right one is slightly larger than the left and is oriented more vertically The primary bronchi subsequently divide into secondary bronchi and then into the tertiary bronchi by week 7.
- By week 24, they divide another 14 times and the respiratory bronchioles have developed.
- They will divide an additional 7 more times before birth.
- As the bronchi develop, the surrounding mesenchyme synthesizes the surrounding cartilages, smooth muscle, connective tissue and capillaries.
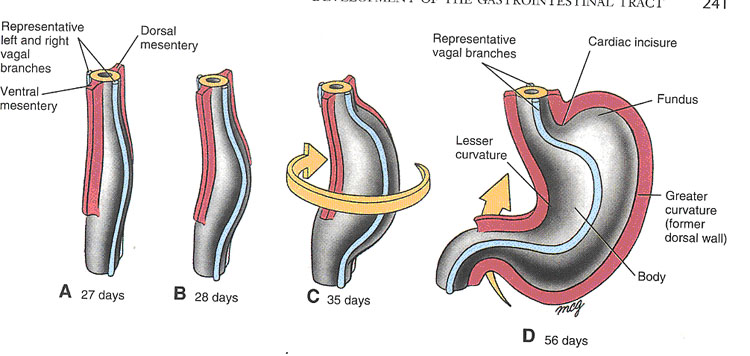
Rotation of the Stomach (Larsen, Fig 9-3)
The developing stomach rotates 90 degrees in a clockwise direction around its longitudinal axis. The original left side becomes the ventral surface, while the original right side becomes the dorsal surface. The greater curvature comes to lie somewhat caudally and to the left; the lesser curvature will be located cranially and to the right. The left vagal trunk will be pulled anteriorly, while the right vagus is pushed posteriorly (Remember the mnemonic “LARP” for left anterior, right posterior)
Creation of the lesser sac (omental bursa)
Rotation of the stomach causes the future duodenum to bend into a “C” shape and to lie against the posterior body wall, so that the mesentery suspending the duodenum fuses with the posterior layer of parietal peritoneum. This process causes duodenum and pancreas to become “secondarily retroperitoneal”. This term is used to differentiate structures which had mesenteries during development, but then lost them, from structures which never had mesenteries at all. The latter structures are termed “primarily retroperitineal” and include kidneys, suprarenal glands, abdominal aorta and inferior vena cava.
Rotation about the longitudinal axis will pull the dorsal mesogastrium to the left, creating a space behind the stomach known as the omental bursa, or lesser peritoneal sac. As this process occurs, the spleen begins to develop within the layers of the dorsal mesogastrium. The spleen, which remains intraperitoneal, is connected to the posterior body wall in the vicinity of the left kidney by the splenorenal (lienorenal) ligament; it is anchored to the stomach by the gastrosplenic (gastrolienal) ligament. These two ligaments are derived from the dorsal mesogastrium
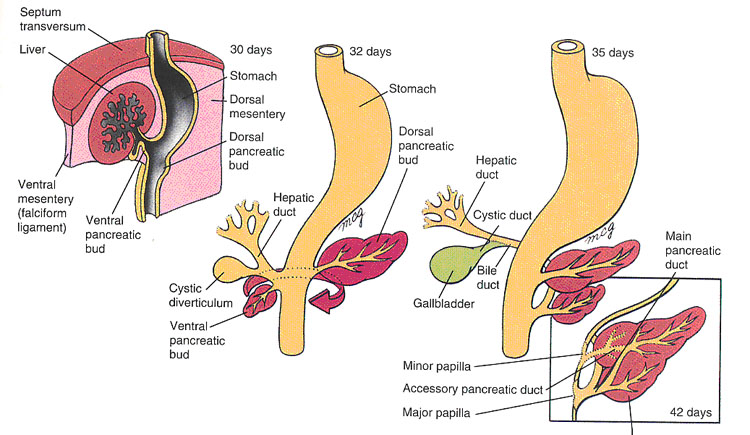
Development of the Pancreas (Larsen, Fig 9-6, 9-7)
Two endodermal buds from the duodenum give rise to the pancreas. The ventral bud arises in the ventral mesentery; the dorsal bud arises in the dorsal mesentery. When the duodenum rotates to the right and assumes its “C” shape, the ventral bud moves dorsally to lie below and behind the dorsal bud. The ventral bud becomes the uncinate process and inferior part of the head of the pancreas. The dorsal bud gives rise to the remainder of the gland.
The main pancreatic duct (of Wirsung) comes from the distal part of the dorsal pancreatic duct and the whole ventral pancreatic duct. The proximal part of the dorsal pancreatic duct may be maintained as an accessory pancreatic duct (of Santorini).
|
Clinical Correlation: Sometimes, the ventral bud splits and wraps around the duodenum. This anomalous migration results in annular pancreas, a ring-shaped portion of pancreatic tissue surrounding the duodenum. It may cause duodenal stenosis. In infants with duodenal atresia, , the digestive tract does not function. Duodenal stenosis, or narrowing, can cause loss of appetite, failure to gain weight, vomiting, a sensation of filling up quickly, or excessive hunger. The condition can also cause a swollen abdomen and colicky, crampy pain. |
Development of the Liver
The liver develops as a diverticulum from the duodenum. It grows toward the septum transversum and eventually fuses with it. The portion of the diverticulum closest to the duodenum develops into the hepatic ducts, the bile duct and the gallbladder. The remaining portion becomes the epithelial plates of the liver. The vitelline veins become incorporated and give rise to the hepatic sinusoids.
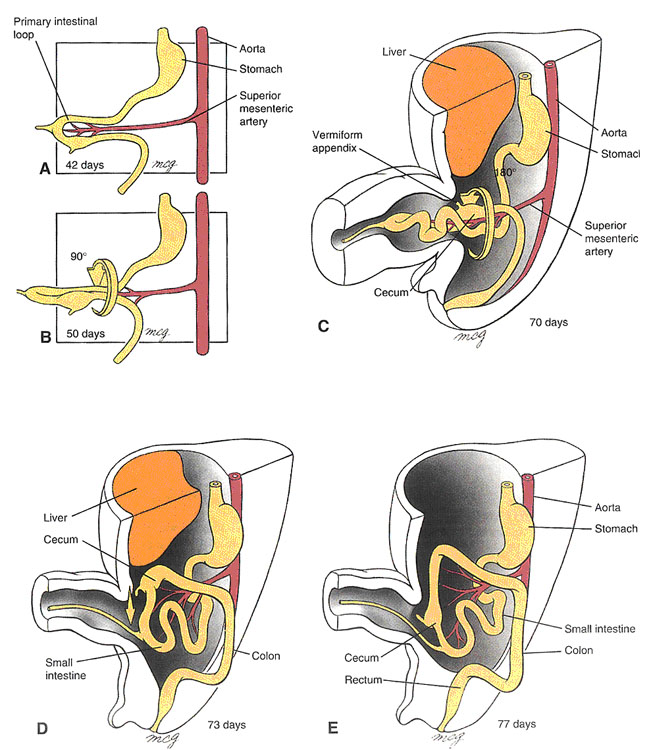
Development of Midgut (Larsen, Fig 9-9)
Midgut development is characterized by rapid elongation of the gut and mesentery. This process occurs at a time when the body cavity is still relatively small, so the primary intestinal loop undergoes a process known as “physiological herniation” into the umbilical cord, beginning at about 6 weeks of development.
The apex of the midgut loops remains in continuity with the yolk sac via the vitelline duct.
During its growth spurt, the intestinal loop rotates around a central axis formed by the artery of the midgut, the superior mesenteric artery. The rotation is counterclockwise, and totals 270 degrees.
By the 10th week, the intestinal loops begin their return to the abdominal cavity. Proximal jejunum is the first to re-enter, and comes to lie on the left side; later returning parts come to lie more and more to the right. The prospective cecum is the last part of the intestinal loop to return to the body cavity. At first, it lies directly below the liver, but from there it normally descends to the right iliac fossa. Failure of this part of the gut tube to descend may result in subhepatic appendix.
As the ascending and descending colon press against the posterior body wall, their mesenteries fuse with the posterior layer of parietal peritoneum, causing these structures to become secondarily retroperitoneal. Abnormalities in midgut rotation can result in conditions such as left-sided colon and reversed rotation of the intestinal loop. Twisting (volvulus) of the gut tube and compromised blood supply can occur.
Occasionally, part of the vitelline duct may persist, forming an ileal (Meckel’s diverticulum)
|
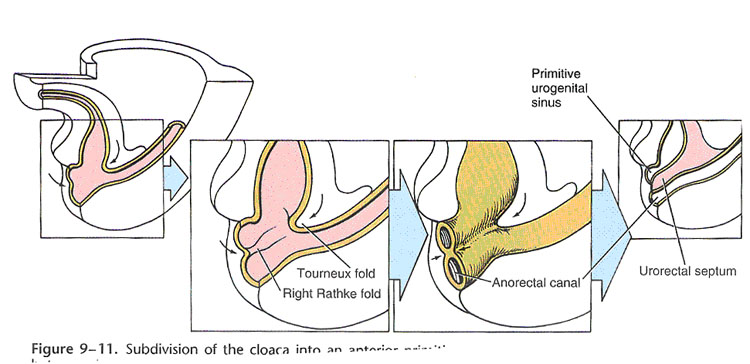
Development of Hindgut (Larsen, Fig 9-11) |
The epithelial lining of the urinary bladder and much of the urethra are also derived from hindgut endoderm. The hindgut terminates in the part of the cloaca which becomes the anorectal canal. The cloaca is an endoderm-lined cavity close off (temporarily) by the cloacal membrane, which contains ecto-and endo-dermal layers but no mesoderm. The cloaca is divided into dorsal and ventral parts by the urorectal septum. This mesenchyme develops in the angle between allantois and hindgut, and separates urogenital sinus from anorectal canal. The urorectal septum fuses with the cloacal membrane to form the perineal body. Most anorectal anomalies result from abnormal partitioning of the cloaca by the urorectal septum.
Clinical CorrelationsEsophageal AtresiaUsually results from abnormal division of the tracheoesophageal septum. The fetus is unable to swallow and this results in polyhydramnios (excessive amount of amniotic fluid) because amniotic fluid cannot pass into the intestines for return to the maternal circulation. Congenital Hypertrophic Pyloric StenosisOvergrowth of the longitudinal muscle fibers of the pylorus creates a marked thickening of the pyloric region of the stomach. The resulting stenosis (Gk. severe narrowing) of the pyloric canal obstructs passage of food into the duodenum, and as a result after feeding the infant expels the contents of the stomach with considerable force (projectile vomiting). This condition affects approximately 1/150 male infants, but only 1/750 female infants. Annular PancreasThe ventral and dorsal pancreatic buds form a ring around the duodenum, thereby obstructing it. Ileal Diverticulum (Meckel’s Diverticulum)A remnant of the proximal part of the yolk stalk that fails to degenerate during the early fetal period results in a finger-like blind pouch that projects from the ileum. While this condition occurs in about 1/50 people, it is usually asymptomic and only occasionally leads to abdominal pain and/or rectal bleeding. OmphaloceleThe midgut fails to retract into the abdominal cavity. At birth, coils of intestine covered with only a transparent sac of amnion protrude from the umbilicus. Malrotations of the MidgutThe midgut does not rotate normally as it retracts into the abdominal cavity. This usually presents as symptoms of intestinal obstruction shortly after birth. Malrotation also predisposes the infant to a volvulus of the midgut, wherein the intestines bind and twist around a short mesentery. Volvulus usually interferes with the blood supply to a section of the intestines, and can lead to necrosis and gangrene. |
Clinical CorrelationsSub-hepatic Cecum and Appendix The cecum and appendix adhere to the inferior surface of the liver during the fetal period, and are carried upwards with it, resulting in an abnormal anatomical position that may create difficulties in diagnosing appendicitis.Stenosis and Atresia of the Small IntestineFailure of recanalization of ileum during the solid stage of development leads to stenosis (narrowing) or atresia (complete obstruction) of the intestinal lumen. Some stenoses and atresias may be caused by an infarction of the fetal bowel owing to impairment of its blood supply (cf. volvulus). Congenital Aganglionic Megacolon (Hirschsprung’s disease)This results from the failure of neural crest cells to migrate and form the myenteric plexus in the sigmoid colon and rectum. The resulting lack of innervation results in loss of peristalsis, fecal retention, and abdominal distention. Anorectal AgenesisAbnormal formation of the urorectal septum causes the rectum to end as a blind sac above the puborectalis muscle. Anal AgenesisAbnormal formation of the urorectal septum causes the rectum to end as a blind sac below the puborectalis muscle. Imperforate AnusThe anal membrane fails to break down before birth. The anus must be reconstructed surgically, with severity depending on the thickness of the intervening tissue. |
Embryology of the Gastrointestinal System quiz click here