13 Embryology of the Heart
EMBRYOLOGY OF THE HEART
Learning Objectives
- Describe the basic embryological development of the heart and the formation of the atria and ventricles and consequences of the malformation of septa and valves
- Describe the fetal circulation and the changes which take place after birth
- Describe the basic formation of the great vessels from aortic arches and their adult derivatives
Reference: Larsen, Human Embryology., 3rd edition, chapters 7 & 8
Particularly relevant Blue Boxes in Moore:
●Variations in the Great Arteries, p. 174
● Embryology of the Right Atrium p. 151
●Septal Defects p. 152
●Valvular Hear Disease p. 153
We also recommend that you look at the excellent tutorials on Cardiovascular Embryology developed by the University of Indiana at:
http://www.indiana.edu/~anat550/cvanim/
and by the University of North Carolina at:
http://www.med.unc.edu/embryo_images/unit-cardev/cardev_htms/cardevtoc.htm
EMBRYOLOGY OF THE HEART
INTRODUCTION
Congenital heart defects are almost all manifestations of improper anatomic develop of the heart, and to understand the resulting dysfunction, its diagnosis, and possible treatment, one must have a firm understanding of the heart’s normal course and pattern of development. Included in the following introductory discussion of this normal development are some of those defects most important to the clinician.
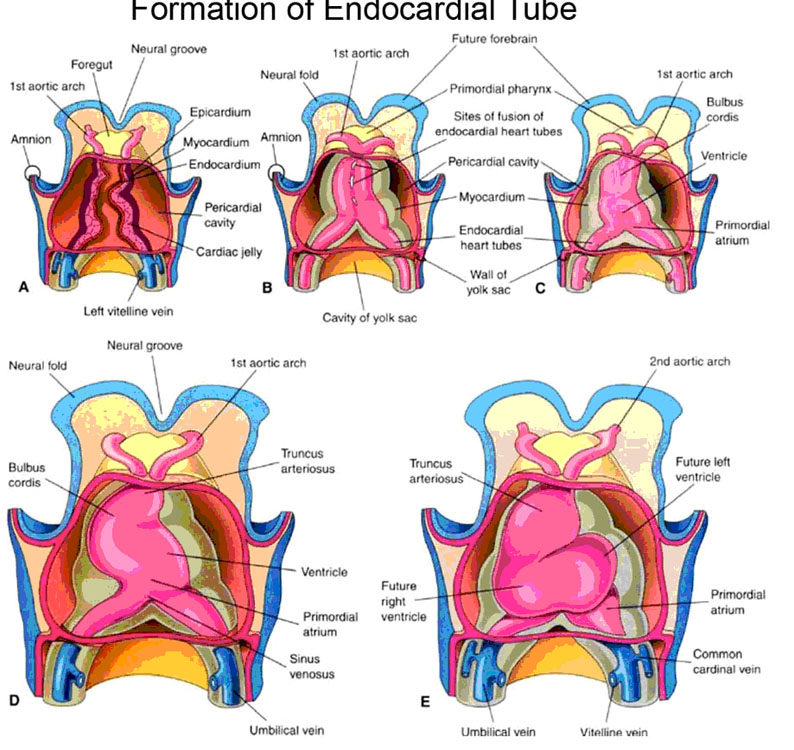
Early in the development of the embryo, two endocardial heart tubes coalesce from angiogenetic cell clusters formed from the splanchnic mesoderm at the cephalic end of the trilaminar disc (Fig. 1). Due to the subsequent lateral folding of the hitherto flat disc, these bilateral endocardial heart tubes are brought next to each other in the midline where they eventually fuse to form the single endocardial heart tube. Simultaneously with lateral flexure, the cephalocaudal flexure takes place and the formerly extreme rostral heart tubes are tucked around and under, to end up ventral and caudal to the bucco-pharyngeal membrane (i.e., in the ‘neck’ area of the embryo.)
It is this single tube that undergoes four basic changes (Fig. 1):
1. “bulge” formation to form the primitive chambers.
2. formation of various longitudinal septi which divide the heart into left and right halves.
3. formation of various transverse divisions that separate the primitive chambers and also form the valves.
4. an anterior, inferior, and leftward “S”-bend.
At the same time as the simple endocardial heart tube is undergoing these changes, it is being cloaked with mesenchymal cells that will eventually form the heart muscle proper (myocardium). The endocardial tube itself becomes the thin inner lining of the heart, the endocardium.
Later in development, when a definitive neck is forming, the heart descends into the thorax to its eventual adult position. The heart’s embryologic relation to the neck explains why many of the cardiac autonomic nerves arise in the neck region, and why the recurrent laryngeal nerves — dragged downward along with the ‘descending’ heart — are forced to ‘recur’ to re-ascend to the neck.
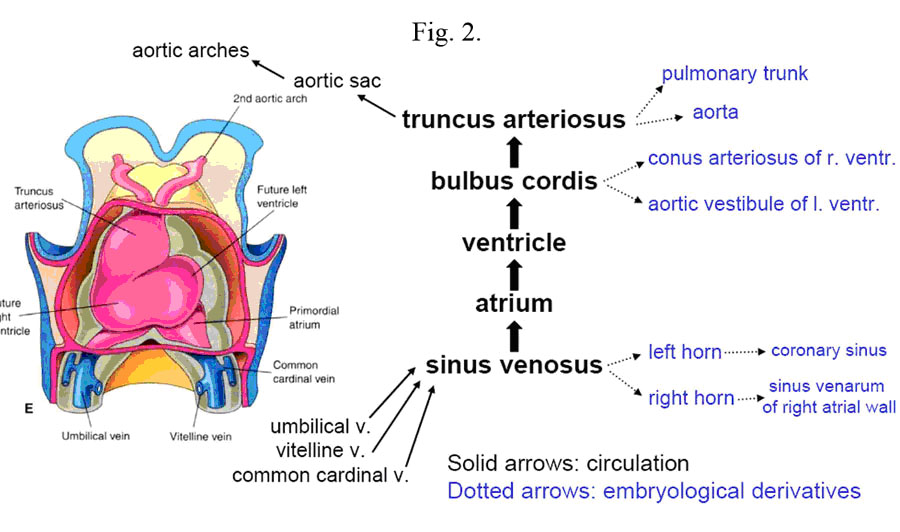
The heart tube contains three specific areas: the cranial portion, the caudal portion and the bulbus cordis. As development progresses, the cranial one-third of the tube dilates to form the aortic sac which will give rise to the aortic arches (Fig. 2).
The caudal one-third to one-half dilates to become the early embryonic ventricle.
The remaining mid portion forms the bulbus cordis which has three areas of development. The proximal one-third gives rise to the body of the right ventricle, which is initially referred to as the primitive ventricle. The remaining two-thirds of the bulbus cordis is divided into two sections. The distal-most section is called the truncus arteriosus which develops into the aortic root and parts of the ascending aorta. The remaining mid-portion is called the conus cordis and connects the primitive right ventricle to the truncus arteriosus. The conus cordis partitions to form the outflow tracts of the right and left ventricles.
An increase in blood volume occurs following the 23rd day of development. With this increase in blood, blood circulation changes to a parallel flow. As the heart tube grows and becomes longer it usually bends to the right.
The heart tube after undergoing S-bending and bulge formation (–>primitive chambers); below are the sections used to show how the various longitudinal, transverse divisions form.
|
● the differentiation of the VENOUS INPUT to the heart. ● the division of the common primitive atria into left and right halves by the INTERATRIAL SEPTUM. ●the separation of the atria from the ventricles and the consequent formation of the atrioventricular valves occurring in the ATRIOVENIRICULAR CANAL. ● the division of the common primitive ventricles into left and right halves by the INTERVENTRICULAR SEPTUM (its two different parts — muscular and membranous — forming separately and differently) .● The division of the TRUNCUS ARTERIOSUS into the pulmonary and aortic outflow tracts, and the formation of the semilunar valves. ●- the differentiation of the complex arteries of the mediastinum from what were originally six simple, paired AORTIC ARCHES . ● and finally, the FETAL CIRCULATION, and how it changes to the adult circulation shortly after birth. |
The simple heart tube undergoes a number of transverse and longitudinal divisions, and some shape changes. Below is discussed:
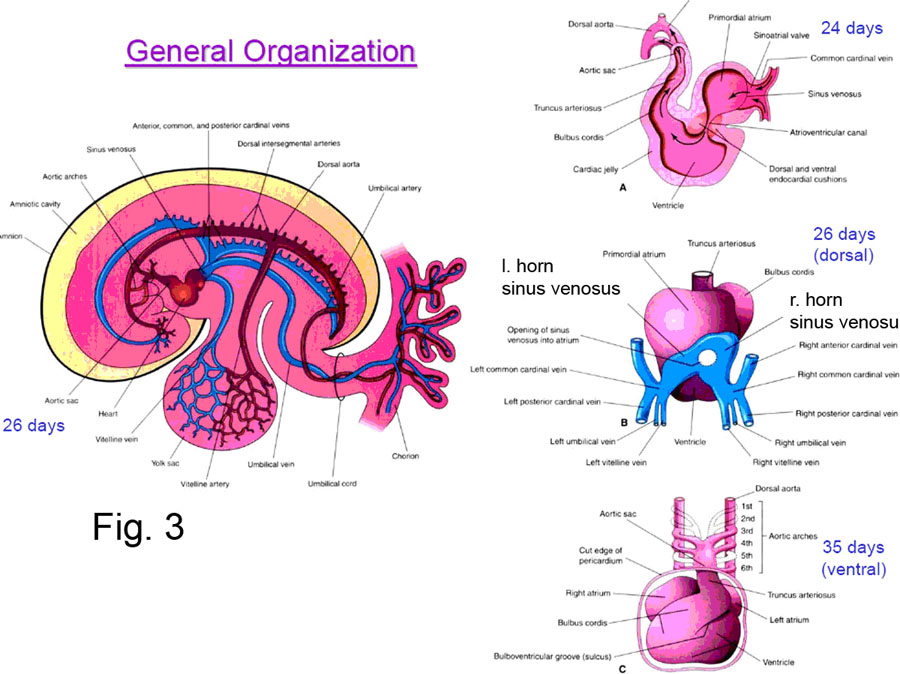
DEVELOPMENT OF THE HEART’S VENOUS INPUT
The original simple venous end of the endocardial heart tube undergoes considerable change to result in those portions of the superior and inferior venae cavae nearest the heart, and the coronary sinus (all emptying into the right atrium); and the 4 pulmonary veins (emptying into the left atrium) (Fig. 3).
The earliest stage of this differentiation results in the formation of the SINUS VENOSUS at the caudal end of the heart tube. All the major veins of the fetal body (vitelline, umbilical, cardinal) drain into the right and left SINUS HORNS, which, empty into the sinus venosus, which empties into the common primitive atria.
With further development and differential growth rates, the opening of the sinus venosus shifts to the right to lie over the developing right atrium.
The RIGHT SINUS HORN becomes incorporated into the right atrium (by a process called intussusception) , giving rise to its smooth walls, and the superior and inferior venae cavae drain into it. The LEFT SINUS HORN remains smaller than the right, and becomes the CORONARY SINUS, receiving the drainage of the coronary veins.
The LEFT ATRIUM, which in the adult should receive the drainage from the lungs, sends out its own common pulmonary vein bud whose branches find their way to the developing lungs. The common vein is then incorporated back into the left atrium (by intussusception), giving rise to its smooth-walled portion; the process of intussusception also producing the four pulmonary veins.
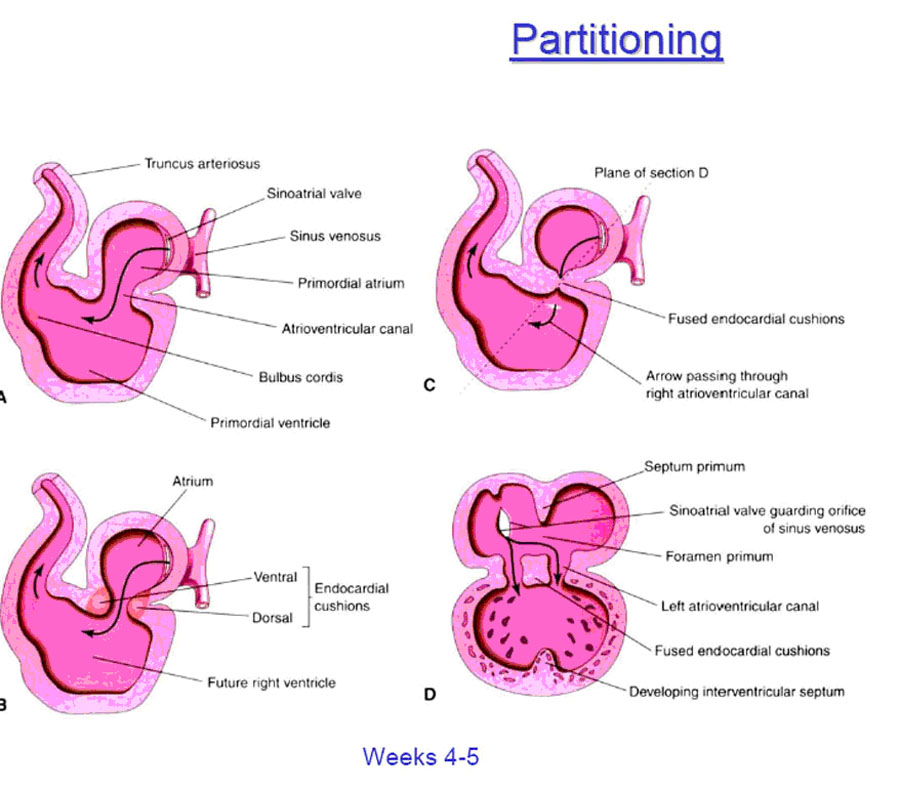
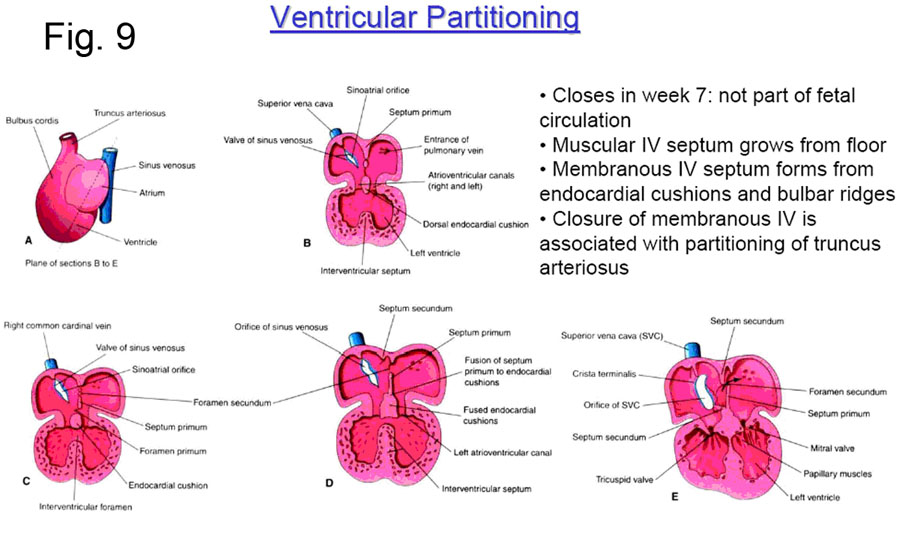
INFLOW SEPTUM AND DIVISION OF THE ATRIOVENTRICULAR CANAL
The primitive atrium empties into the primitive left ventricle through the atrioventricular canal (Fig. 4). As development progresses endocardial cushions then appear around the edges of the atrioventricular orifice. These are the precursors of the atrioventricular valves and function during this early development as primitive valves. The dorsal and ventral endocardial cushions grow toward each other and fuse, thus separating the atrioventricular canal into two openings which will eventually become the tricuspid and mitral valves. This separation occurs simultaneously.
FORMATION OF THE INTERATRIAL SEPTUM
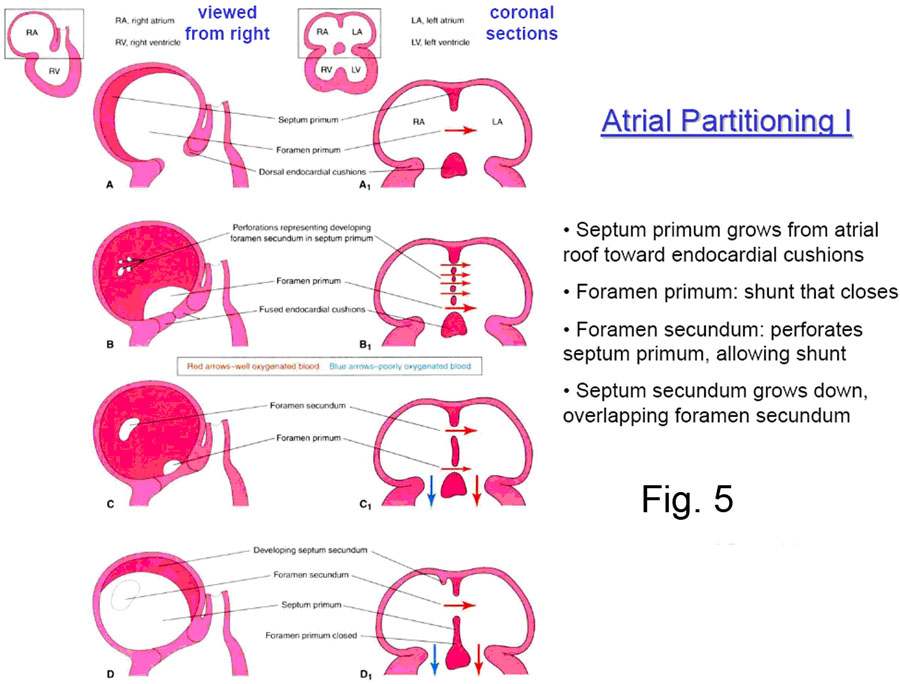
The primitive common atrial chamber is divided into left and right halves by the formation of the interatrial septum. In the embryo, this septum is formed by two sequentially appearing and adjacent septae that later fuse together to form the single layer of the adult structure.
The two embryonic septi are the SEPTUM PRIMUM and the SEPTUM SECUNDUM
(Fig. 5). The septum primum — first to appear — one time or another has two holes through it; first the OSTIUM PRIMUM, followed later by the OSTIUM SECUNDUM. The septum secundum has only one hole in it, the FORAMEN OVALE.
The SEPTUM PRIMUM first appears in the roof of the common atrium, and begins to grow down towards the endocardial cushions ringing the atrioventricular canal (Fig. 6).
As the inferior edge of the septum primum approaches near the ingrowing endocardial cushions, a hole –allowing for continued communication between the newly divided left and right atria — is defined, and this is the OSTIUM PRIMUM. Normally, it is soon closed by the continued ingrowth of the endocardial cushions.
However, before the ostium primum has even finished being closed off, the septum primum undergoes localized cell death superiorly to form a hole known as the FORAMEN SECUNDUM. This continued communication between the right and left atria is crucial to the fetus, because until the baby is born, blood must be able to pass from the right atrium into the left. (See ‘The Fetal Circulation’).
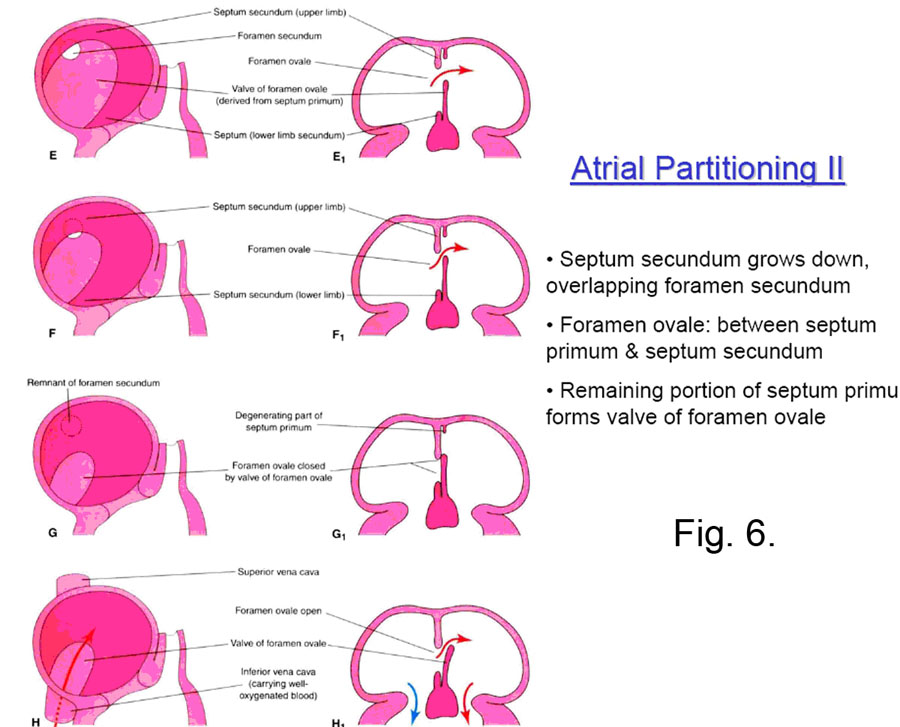
At about this time the SEPTUM SECUNDUM begins to grow down from the right atrial roof immediately adjacent to the septum primum. It never completely blocks off the interatrial communication, because its inferior concave edge only descends to the posteroinferior aspect of the right side of the septum primum, leaving the FORAMEN OVALE. This foramen opens to the left atrium by a flap valve formed by the septum primum (see Fig. 7)
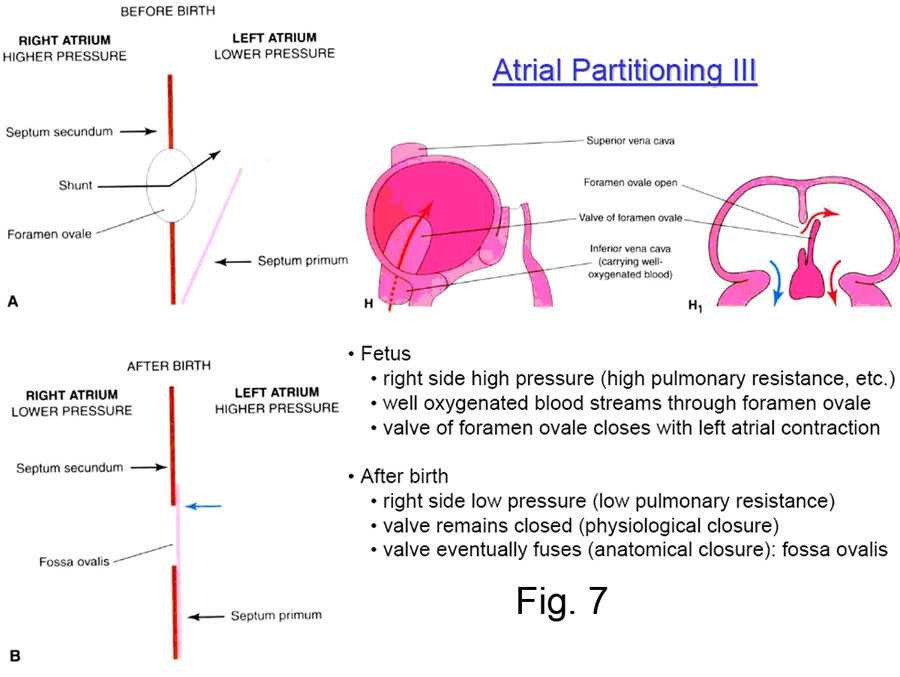
Systemic blood in the fetus reaching the right atrium through the inferior vena cava is shunted by the valve of the inferior vena cava in large part through the foramen ovale and into the left atrium and thus back to the body (Fig. 7).
|
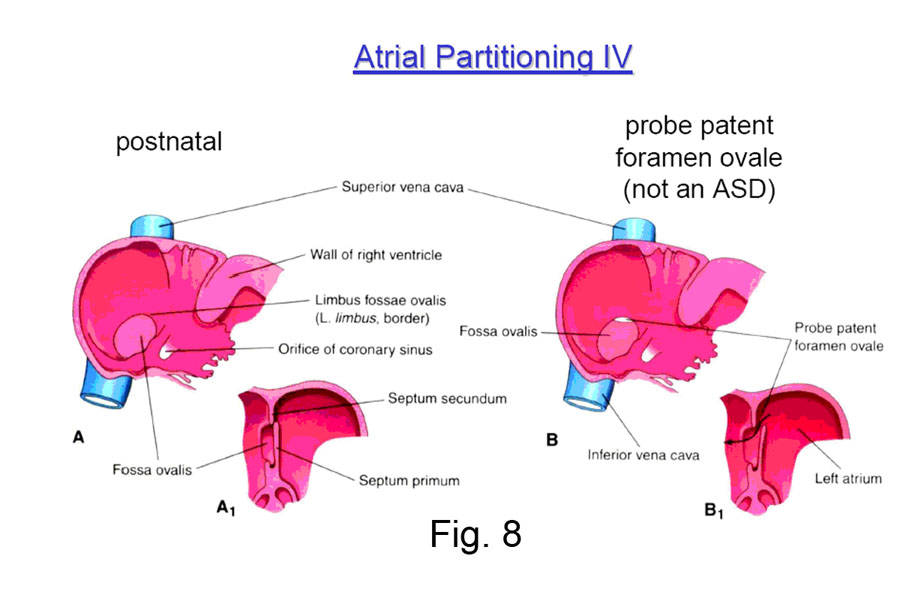
Clinical Note: When the child is born, the pressure in the right atrium drops — because no more blood is returning to it from the placenta — and the pressure in the left atrium rises — because blood begins to flow in quantity through the newly aerated lungs and return to the left atrium. The important result is that the septum primum is forced strongly against the septum secundum, and the interatrial communication is functionally closed. After some months the two septi then grow together permanently in some 75-80% of all cases; in the other 20-25%, they are still anatomically open (probe patent) but functionally closed by the pressure difference (Fig. 8). |
|
Clinical Note: If the ostium secundum and the foramen ovale directly overlap, then the communication between the left and right atria remains open after birth. This results in blood from the left atrium pouring into the right atrium, which is not an ideal situation. Since this defect involves the ostium secundum, it is known as a OSTIUM SECUNDUM type of atrial septal defect. If the ostium primum fails to close, the resultant persisting communication is often called an OSTIUM PRIMUM type of defect. The closure of the ostium primum is afforded by the endocardial cushions surrounding the atrioventricular canal, which also form the atrioventricular valves and part of the (membranous) interventricular septum. There is a whole class of defects of varying manifestations resulting from improper growth of the endocardial cushion — known as ENDOCARDIAL CUSHION DEFECTS — of which ostium primum defects are just a part. |
FORMATION OF THE INTERVENTRICULAR SEPTUM
The two parts of the interventricular septum — membranous and muscular — are formed separately.
The MUSCULAR SEPTUM forms by apposition of the adjacent walls of the two growing ventricles (Fig. 9). The ventricles begin to grow quite rapidly by a hollowing out and trabecula formation on the inside, and the continuous deposition of new muscle on the outside. This results in a soft spongy flexible heart; if the inner trabeculation did not occur, the heart would shortly become too thick and stiff to adequately contract. After a while, the ventricles are almost totally separated by this muscular septum and the small communication superior to the muscular septum is the INTERVENTRICULAR FORAMEN This will later close by the formation of the membranous portion of the interventricular septum.
The MEMBRANOUS SEPTUM forms after the completion of the muscular septum; partially by the growth of the inferior endocardial cushion, and partially from the downward continuation of the spiral septum (next section) that divides the arterial outflow tracts of the truncus arteriosus.
The left ventricle forms largely from the primitive ventricle, while the right ventricle forms largely from the bulbus cordis.
This occurs due to the relative shift of the truncus arteriosus over to the left side, so that the left ventricle has direct access to the truncus outflow tracts, and the bulbus and conus are shifted off to the right side. The smooth walled conus contributes to the INFUNDIBULUM — the smooth superior outflow portion of the adult right ventricle.
If the membranous portion of the interventricular septum does not completely close the interventricular foramen, the ensuing communication between the ventricles is called a VENTRICULAR SEPTAL DEFECT.
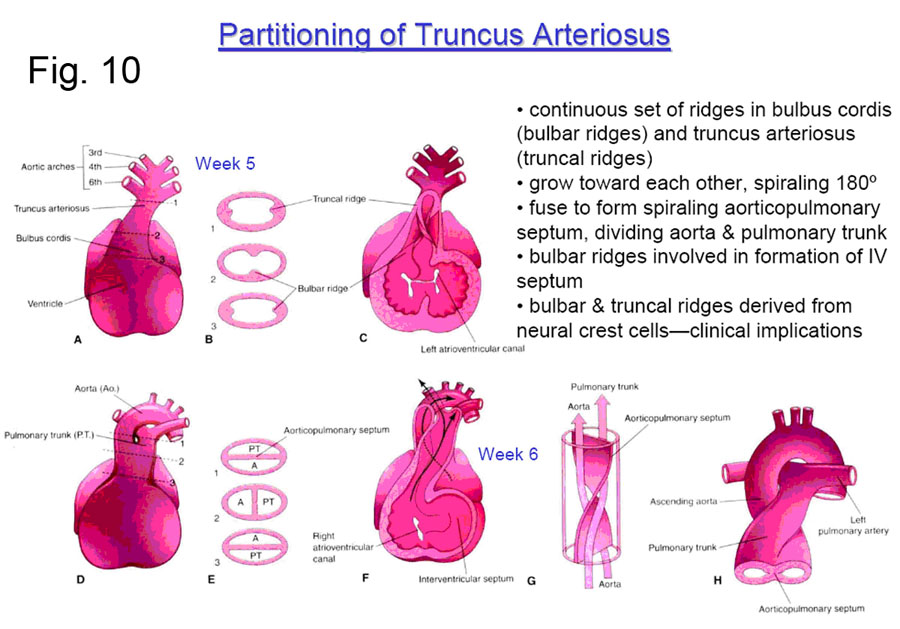
DIVISION OF THE TRUNCUS ARTERIOSUS
The original common arterial outflow tract — the TRUNCUS ARTERIOSUS —is soon divided into aortic and pulmonary portions by the formation of the TRUNCOCONAL (aorticopulmonary) SEPTUM (Figure 10). This septum is formed by ridges which swell along the inner aspect of the truncus and then grow inward to meet in the middle. These ridges also form the precursor swellings which will become the SEMILUNAR VALVES; the truncal ridges are derived from neural crest cells.
Note that the truncoconal septum has a spiral septum, which achieves the crossing of the arterial outflow tracts (aortic and pulmonic). This septum also forms most of the membranous portion of the interventricular septum (with the help of the inferior endocardial cushion.).
A variety of outflow tract malformations result from errors in the septation of the truncoconal septum. Errors in this process may be caused by abnormal neural crest cell migration. In about 1 in 10,000 infants, the truncoconal septa do not form at all, resulting in a persistent truncus arteriosus. This malformation necessarily includes a ventricular septal defect. The result is that blood from the two sides of the heart mixes thoroughly in the common outflow tract, and both the body and the lungs receive partially deoxygenated blood. Untreated infants usually die within 2 years.
In about 5 of 10,000 infants, the truncoconal septa do not display the usual spiral pattern. The result is transposition of the great vessels in which the left ventricle empties into the pulmonary circulation and the right ventricle empties into the systemic circulation. This condition is not immediately fatal because the deoxygenated systemic blood and the oxygenated pulmonary blood can mix through a patent foramen ovale or ductus arteriosus. Nevertheless, it is the leading cause of death in infants younger than 1 year old with cyanotic heart disease.
|
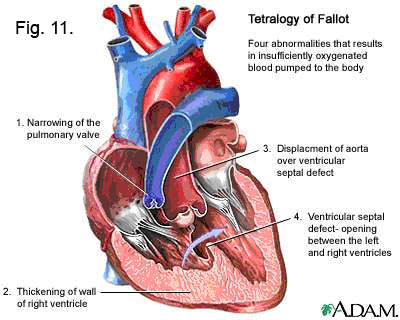
Clinical Note: One of the most common congenital defects — resulting from the displacement of the truncoconal septum too far to the pulmonary side of the truncus arteriosus — is known as the TETRALOGY OF FALLOT (Fig. 11). The truncoconal septum shift results in a small pulmonary trunk and/or small pulmonic valve; and an “Overriding” aorta, which now extends over the right ventricle where the pulmonary trunk should have been. In addition, the truncoconal septum, hopelessly out of place, cannot contribute to the formation of the membranous portion of the interventricular septum and thus there is a ventricular septal defect. And finally, since the right ventricle thereby gets lots of extra blood from the left ventricle — and blood at the higher pressure of the left ventricle — it hypertrophies ( = enlarges to cope with the extra load.)
|
SUMMARY
CARDIOVASCULAR EMBRYOLOGY
The cardiovascular system begins to develop during week 3.
Mesenchymal cells derived from the mesoderm form endothelial tubes which join to form the primitive vascular system
HEART DEVELOPMENT (WEEK 3)
Heart develops from splanchnic mesenchyme in the cardiogenic area.
Bilateral cardiogenic cords
- are formed from the mesenchyme
- become canalized
- and form the paired endocardial heart. These fuse into a single heart tube forming the primitive heart.
Surrounding mesenchyme thickens to form the myoepicardial mantle (future myocardium and epicardium) separated from the endothelial heart tube (future endocardium) by the gelatinous cardiac jelly
The future heart develops dilatations and constrictions resulting in 4 chambers
- sinus venosus
- primordial atrium
- ventricle
- bulbus cordis
The truncus arteriosus is continuous caudally with the bulbus cordis, and enlarges cranially to form the aortic sac from which the aortic arches arise
The sinus venosus receives :
- the umbilical veins from the chorion.
- the vitelline veins from the yolk sac
- the common cardinal veins from the embryo.
3 systems of paired veins drain into the primitive heart:
- the vitelline system will become the portal system;
- the cardinal veins will become the caval system;
- the umbilical system which degenerates after birth.
The bulbus cordis and the ventricle grow faster and the heart bends upon itself, forming a bulboventricular loop.
The atrium and sinus venosus come to lie dorsal to the bulbus cordis, truncus arteriosus and ventricle.
At the same time, the heart invaginates into the pericardial cavity.
The dorsal mesocardium which attaches it to the dorsal wall of the pericardial cavity degenerates and forms the tranverse pericardial sinus.
First heartbeat occurs at 21 to 22 days and originates in the muscle, forming peristalsis-like waves beginning in the sinus venosus.
By the end of week 4 coordinated contractions of the heart results in unidirectional flow:
- Blood enters the sinus venosus from the vitelline, cardinal and umbilical veins
- Blood flows into the primitive ventricle;
- Upon ventricular contraction, blood flows into the bulbus cordis and the truncus arteriosus into the aortic sac, passing into the aortic arches and branchial arches;
- Blood then passes to the dorsal aortae for distribution to the embryo, yolk sac and placenta.
The heart divides into 4-chambered heart between weeks 4 and 7.
1) Endocardial cushions form on the dorsal and ventral walls of the atrioventricular canal. At week 5, they approach each other and fuse, dividing the atrioventricular canal into right and left canals.
2) Atria are partitioned successively by the septum primum and the septum secundum . The latter is an incomplete partition and leaves a foramen ovale. The foramen ovale has a valve formed from the degeneration of the cranial portion of the septum primum.
Before birth the foramen ovale allows blood to pass from the right atrium into the left atrium; reflux is prevented by the valve.
After birth the foramen ovale normally closes by fusion of the septum primum and the septum secundum.
3) The sinus venosus develops a left horn which becomes the coronary sinus and a right horn which will be incorporated into the right atrium. The smooth part of the right atrium, the sinus venarum, is derived from the sinus venosus whereas the muscular part, the auricle, is derived from the primitive atrium.
4) The primitive pulmonary vein and its 4 main branches become partially incorporated into the left atrium. This results in the 4 pulmonary veins.
5) The ventricles become partitioned by a crescentic fold which is open cranially until the end of week 7 (interventricular foramen; .The interventricular septum is formed of a central membranous part and a surrounding muscular part. After closure, the right ventricle communicates with the pulmonary trunk and the left ventricle with the aorta.
6) During week 5, the bulbus cordis and the truncus arteriosus become divided by an aorticopulmonary septum into the definitive pulmonary trunk and aorta. Valves develop from proliferation of the subendocardial tissue.
The primitive atrium acts as a temporary pacemaker. But the sinus venosus soon takes over.
- The sinoatrial (SA) node develops during week 5. It is part of the sinus venosus which becomes incorporated into the right atrium.
- The atrioventricular (AV) node also develops from the cells in the wall of the sinus venosus together with cells from the atrioventricular canal region.
The critical period of development is from day 20 to day 50 after fertilization.
Improper partitioning of the heart may result in defects of the cardiac septa, of which the ventricular septal defects are most common (25% of congenital heart disease).
Membranous ventricular septal defect (most common):
- involves the oval membranous portion of the interventricular septum which fails to develop.
- is due to the failure of extensions of subendocardial tissue growing from the right side of the fused endocardial cushions and fusing with the aorticopulmonary septum and the muscular part of the interventricular septum.
Muscular septal defect:
- Perforation may appear anywhere in the muscular part of the interventricular septum (multiple defects = Swiss cheese type of ventricular septal defect) due perhaps to excessive resorption of myocardial tissue during formation of the muscular part of the interventricular septum.
The tetralogy of Fallot consists of .
- pulmonary valve stenosis: the cusps of pulmonary valve are fused together to form a dome with a narrow central opening.
- ventricular septal defect
- overriding aorta
- hypertrophy of right ventricle
- Cyanosis is an obvious sign but may not be present at birth.
FETAL CIRCULATION
THE FETAL CIRCULATION (Netter 226)
The basic differences in the fetal circulation from the adult circulation is that the fetus receives oxygen from the placenta, the vascular structure outside its body which it must reach via the umbilical vessels. Unlike the adult — which receives oxygen by exchange with in the lungs — the fetus has small dense fluid filled lungs which admit little blood. So the basic problem is that the right heart and its connections to the lung must be fully developed and ready to go at the instant of birth, but must be totally non-functional “in utero”.
● Oxygenated blood returns from the placenta by the umbilical vein.
- Half of the blood passes through the liver whereas the other half bypasses the liver by the ductus venosus.
- Blood enters into the inferior vena cava and then the right atrium of the heart. This blood is now partially deoxygenated because it is mixed with returning blood from the lower portion of the body and the abdominal organs.
- Most of the blood in the right atrium passes through the foramen ovale into the left atrium and mixes with the small amount of blood returning from the lungs (deoxygenated).
- From the left atrium, blood passes into the left ventricle and the ascending aorta. Arteries to the heart, head and neck, and upper limbs receive well-oxygenated blood.
- A small amount of blood from the right atrium mixes with blood from the superior vena cava and coronary sinus. It passes into the right ventricle and leaves via the pulmonary trunk. Most of it passes into the ductus arteriosus into the aorta. A small amount passes into the lungs.
- 50% of the blood passes via the umbilical arteries into the placenta for reoxygenation, the rest supplies the viscera and the inferior 1/2 of the body.
After birth, the foramen ovale, ductus arteriosus, ductus venosus and umbilical vessels are no longer needed and they close .
The right ventricular wall is thicker in the newborn but by the end of month 1, the left ventricular wall is thicker.
The fetal circulation is designed to carry oxygenated blood from the placenta to the fetal circulation, bypassing the lungs.
- Changes that will result in a normal adult circulation occurs during infancy.
- Defects will commonly involve a patent foramen ovale and/or patent ductus arteriosus