1 Anatomy and Physiology of the Hypothalamus and Pituitary
Brent Cochran, PhD & Arthur Tischler, MD
Learning Objectives
- Contrast the anterior and posterior pituitary lobes with respect to cell types, vascular supply, and innervation.
- List the target organs and cell types for oxytocin and vasopressin, and describe their effects on each.
- Describe the stimuli for secretion of vasopressin and oxytocin.
- Identify disease states caused by the over production and under production of vasopressin, and list the principle symptoms of each.
- Describe the synthesis, structure and actions of the anterior pituitary hormones including GH, PRL, LH, FSH, TSH, ACTH, α-MSH, and β-endorphin. Describe the principle effects of under secretion and over secretion of each.
- Identify the hypothalamic hormones that control secretion of the anterior pituitary hormones.
- Explain the principles of negative feedback control and of positive feedback control of hormone secretion. Explain the difference between long loop, short loop, and ultrashort loop negative feedback.
- Diagram the short loop and long loop negative feedback secretory control of the anterior pituitary hormones.
- Describe the importance of pulsatile and diurnal patterns of hypothalamic and pituitary hormone release.
Key Concepts
- The major hormones produced by the anterior pituitary are GH, TSH, LH, FSH, ACTH and prolactin. The hormones produced by the posterior pituitary are ADH(vasopressin) and oxytocin.
- Diabetes insipidus is most often caused by insufficient ADH production or
responsiveness. - Anterior pituitary function is under regulation by the hypothalamus.
- Anterior pituitary hormone release is under feedback inhibition by
peripherally produced hormones. - Effects of GH on bone growth are mediated by IGF-1 produced by the liver.
- Only prolactin secretion is under negative control by the hypothalamus. The
other anterior pituitary hormones are under positive control by releasing
hormones.
Anatomy/Histology
Embryology
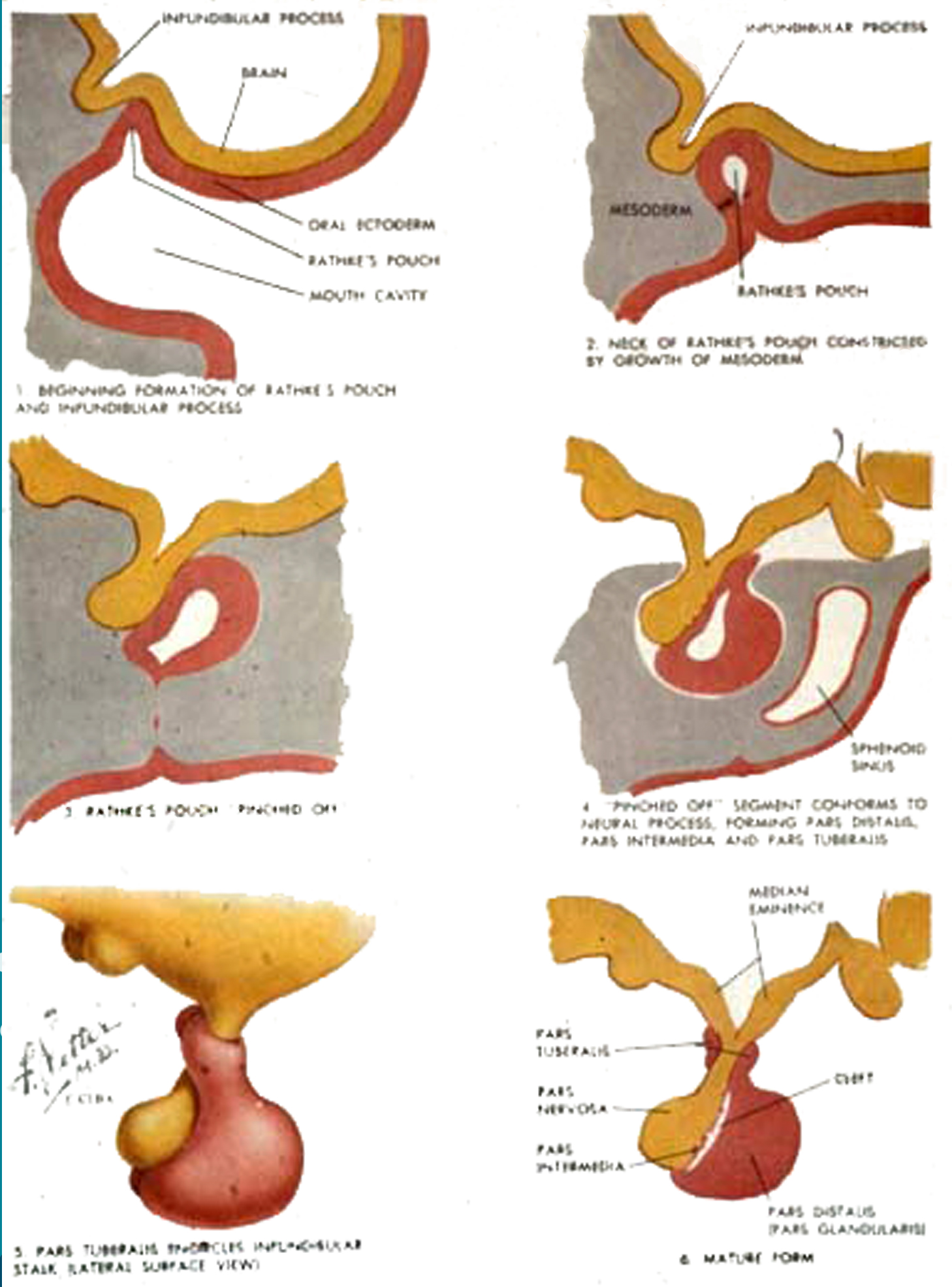
The pituitary is derived from two separate primordia: (1) an upward invagination of oral ectoderm, known as Rathke’s pouch, from the primitive pharynx, or stomodeum, and (2) a downward growth of neural ectoderm, the infundibulum, from the floor of the developing hypothalamus. In the human embryo Rathke’s pouch forms at about three week’s gestation. By about two months it separates from the oral cavity and comes in contact with the infundibulum. Subsequently, the infundibulum gives rise to the neurohypophysis (the posterior pituitary), and Rathke’s pouch to the adenohypophysis (the anterior pituitary). A remnant of Rathke’s pouch normally persists as a glandular rest, the pharyngeal pituitary, in the midline in the roof of the nasopharynx.
At the molecular level, specific transcription factors have been associated with the development of each cell type in the adenohypophysis. Mutations of these factors can on occasion cause selective insufficiency of pituitary hormones with no insufficiency of the hormone genes themselves. The factors have also been used as surrogate markers to identify the lineage of non-functional tumors.
Gross Anatomy, Vascular Supply and Innervation
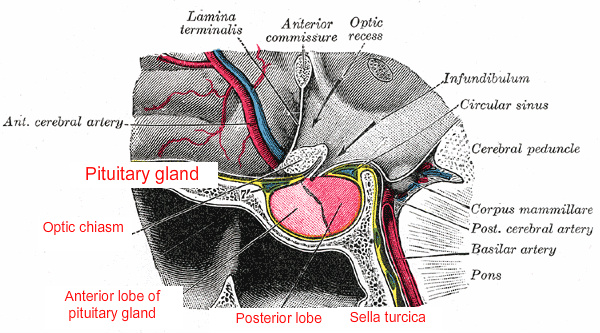
The pituitary is attached by its stalk to the underside of the brain, just behind the optic chiasm. Because of this location, the ancient Greeks referred to it as the hypophysis cerebri (hypo, under + physis, growth), in contrast to the epiphysis cerebri, the pineal gland. It is protected by its location in the hypophyseal fossa of the sella turcica, a cavity in the sphenoid bone, which is partially covered by the diaphragma sellae, an extension of dura mater.
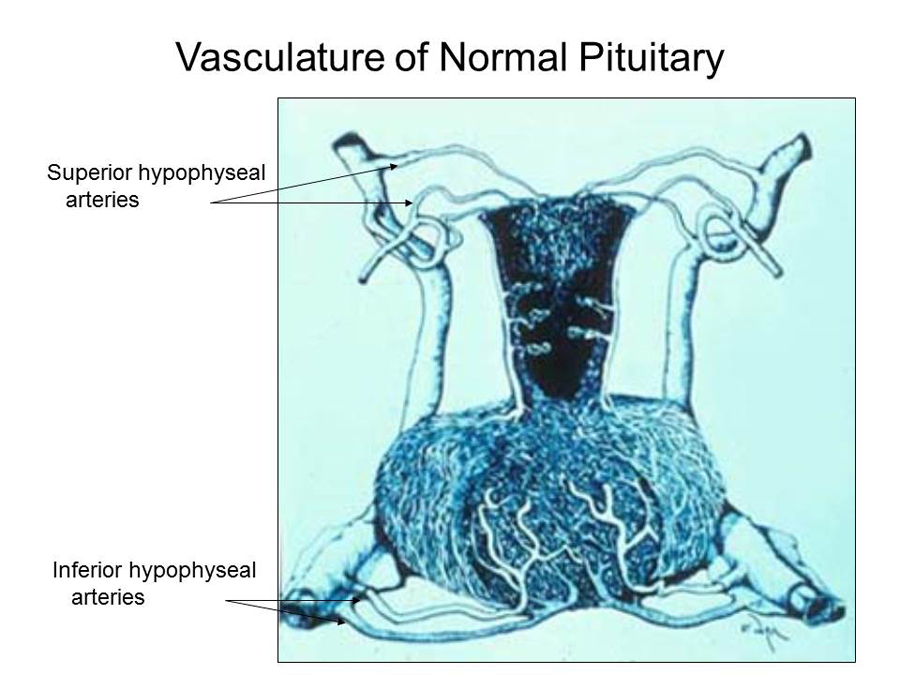
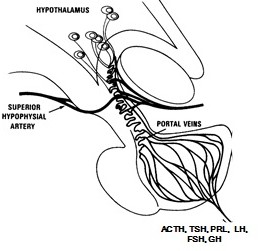
The pituitary blood supply is derived from paired superior and inferior hypophyseal arteries, originating respectively from the internal carotid arteries in the circle of Willis and in the cavernous sinuses. The adenohypophysis derives most of its blood from the superior hypophyseal artery by way of a portal venous system that delivers hypophysiotrophic hormones released by hypothalamic secretory neurons. The neurohypophysis is supplied with blood directly from branches of these arteries. The inferior hypophyseal arteries, which enter the floor of the hypophyseal fossa, are not part of the hypophyseal-portal system. Venous blood from both lobes of the pituitary empties into the cavernous sinuses from a number of small veins.
The neurohypophysis has direct axonal connections with two major nuclei in the hypothalamus, the supraoptic and paraventricular nuclei. In contrast, the nerve supply of the adenohypophysis is limited to a few fibers from the carotid plexus, which probably have a vasomotor function.
 Function and Cytology of the Normal Pituitary
Function and Cytology of the Normal Pituitary
The Greek physician Galen (AD 130 200) believed that the function of the hypophysis cerebri was to extract mucus from the brain and to secrete it into the nose and pharynx. This view persisted into the 17th century and led Vesalius (1514 1564) to coin the term pituitary from the Latin root pituita, meaning phlegm.
We now know that the adenohypophysis produces six major hormones: Growth hormone (GH, also known as somatotrophic hormone), prolactin, ACTH, thyrotropin (TSH), Luteinizing hormone (LH), and follicle stimulating hormone (FSH).
In routine histological sections stained with hematoxylin and eosin, three cell types can be recognized:
- Acidophils (red, stain with eosin, an acidic dye), about 40%
- Basophils (purple, stain with hematoxylin, a basic dye), about 10%
- Chromophobes, about 50%
Correlation of hormone production and cytology began with attempts to subclassify the acidophils and basophils. Those efforts first relied on special stains (which also led to a reduction in the number of “chromophobes”), and then on immunocytochemistry and electron microscopy. Although some studies attempted to associate production of specific hormones with the presence of granules of specific size and electron density, other investigations presented evidence that granule size varies with levels of secretory activity; i.e., that actively secreting cells contain smaller granules than less actively secreting cells, and that granule size is not a reliable criterion for hormone identification. For most purposes adenohypophyseal cell types are now characterized by immunohistochemistry. However, the heterogeneous H&E staining pattern is useful to pathologists for discriminating normal pituitary from pituitary adenomas, which have a homogeneous cell population.
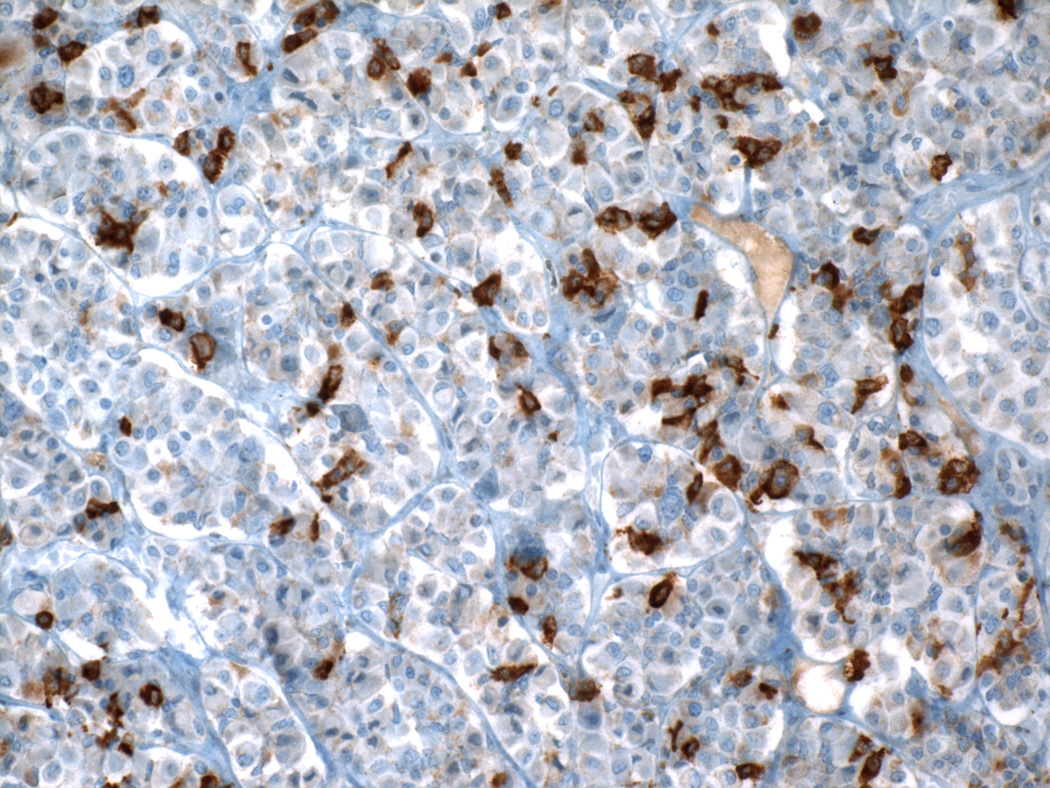
Two separate acidophil cell types are now known to produce GH (about 50% of all cells), and prolactin (about 15% of all cells). A third acidophil type may produce both GH and prolactin. Two separate basophils produce ACTH (about 15 20% of all cells) and TSH (less than 10% of all cells); and a third basophil is a combined “gonadotroph” cell producing both FSH and LH (about 10% of all cells). Electron microscopy shows that most of the cells classified as chromophobes by light microscopy contain a few secretory granules similar to those in chromophil cell types, suggesting that they are in fact functional cells in a degranulated stage of a secretory cycle. Specific adenohypophyseal cell types undergo morphological changes in response to physiological stimuli (e.g., “Crooke’s hyaline change”); circumnuclear cytoplasmic clearing visible by light microscopy in corticotrophs after sustained elevation of glucocorticoid blood levels, corresponding ultrastructurally to accumulations of 10 nm filaments of the keratin family.
The neurohypophysis does not contain its own secretory cells. Instead, it is composed of glial cells and axons of secretory neurons that have their cell bodies in the hypothalamus. The neurons in these nuclei synthesize vasopressin, oxytocin, and related small peptides, and transport them directly along their axons to the neurohypophysis where they accumulate in and around axon terminations, eventually to enter the peripheral blood.
Physiology
Introduction
The pituitary produces hormones that regulate the synthesis and secretion of several other hormones and control the overall growth of the organism. The pituitary weighs only one gram in an adult human. It is composed of a posterior and an anterior lobe. A summary of these hormones and their major actions on target tissues is in Table 1.
| Table 1: Pituitary hormones and their actions. | |||
| HORMONE | STIMULUS | TARGET TISSUES | ACTIONS |
| Posterior Pituitary | |||
| ADH | ↓Py↑Posm | kidney collecting duct | ↑permeability to H2O |
| Oxytocin | CNS | breast uterus |
↑smooth muscle contraction |
| Anterior Pituitary | |||
| growth hormone | sleep, exercise, hypoglycemia | many tissues adipose, liver |
growth lipolysis |
| Prolactin | breast stimulation, CNS | breast ovaries |
milk production ↑maternal blood glucose |
| TSH | low temperature | thyroid | ↑T3 and T4 |
| LH | CNS | ovaries testes |
estrogens ↑androgens |
| FSH | CNS, activin, inhibin | ovaries testes |
oocyte maturation sperm maturation |
| ACTH | stress | adrenal cortex | ↑glucocorticoids |
| MSH | ? | skin | melanin production |
| Endorphins | CNS, stress | sensory neurons | ↓pain sensitivity |
The Posterior Pituitary (Neurohypophysis)
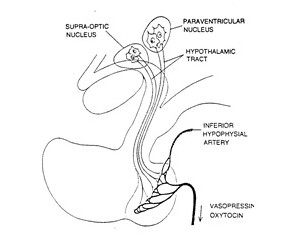
Hormones secreted by the posterior pituitary are synthesized by neurosecretory cells whose cell bodies actually lie in the hypothalamus (Figure 1). Therefore, removing the pituitary will result in transient absence of ADH and oxytocin, but these hormones will return when the axons regenerate and reach new capillary beds.
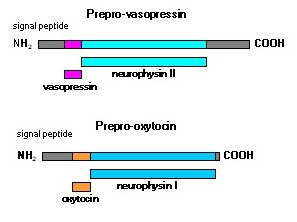
Antidiuretic hormone (ADH; also called vasopressin.) and oxytocin are synthesized as much larger precursor proteins (Figure 2). They are packaged into secretory vesicles along with carrier proteins called neurophysins and are then transported to the axon termini in the posterior pituitary.
Increased plasma osmolarity or decreased plasma volume will cause ADH release. ADH increases the permeability of the kidney collecting duct to water by stimulation of insertion of aquaporins into the membrane so that more water is reabsorbed. Loss of ADH expression results in diabetes insipidus with extreme levels of dilute urination along with an increase of thirst. In addition, ADH is a vasoconstrictor as it stimulates contraction of vascular smooth muscle cells. Decreases in blood pressure are detected by baroreceptors in the heart which signals AVP release through the vagus and glossopharyngeal nerves. ADH secretion is more sensitive to changes in osmolality than blood pressure and is mediated by osmoreceptive neurons in the hypothalamus which innervate the magnocellular neurons that produce ADH. Tumors that produce excess ADH will lead to increases in blood volume and pressure along with increases in urine osmolality along with low blood sodium (hyponatremia) due to the stimulation of atrial natriuretic peptide (ANP) production. This is known as the syndrome of inappropriate secretion of antidiuretic hormone (SIADH). ADH can also be elevated in primary hypoadrenalism cases.
Oxytocin release is triggered by breast and nipple stimulation or the sound of a baby crying. It causes contraction of smooth muscle cells in the breast to force milk from higher ductules into larger collecting ducts. Oxytocin also stimulates smooth muscle contraction in the uterus, and oxytocin derivatives are commonly used to induce labor. Oxytocin may also promote social cognition and empathy in humans.
The Anterior Pituitary
Neurosecretory cells in the hypothalamus release hormones into a portal circulation between the hypothalamus and anterior pituitary (Figure 3). These hypothalamic hormones regulate the synthesis and secretion of all hormones of the anterior pituitary.
The hormones in the anterior pituitary can be conveniently grouped into 3 classes.
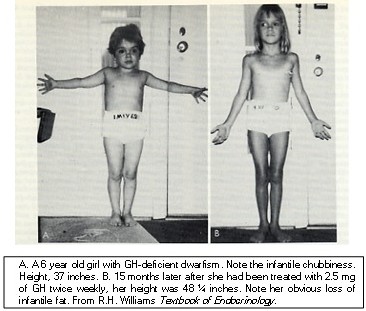
(1) Growth hormone-related hormones include GH and prolactin (PRL). These hormones are >80% identical in their amino acid sequences. They are approximately 22 kDa in size. A third hormone in this family is placental lactogen. Growth hormone promotes growth and differentiation of many different tissues. Hepatocytes contain GH receptors, and in the presence of GH, the liver secretes insulin-like growth factor-1 (IGF-1). There is also GH induced IGF-1 release locally in the bones. The growth of most tissues in response to GH is actually caused by the actions of IGF-1. GH excess in childhood leads gigantism (Figure 4).


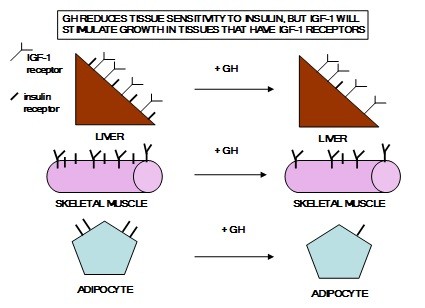
GH excess in adults leads to acromegaly (Figure 5). GH causes muscle, heart, liver, and adipose tissue to become less responsive to insulin. However, most tissues will take up amino acids and grow under the influence of IGF-1. GH causes lipolysis in adipose tissue by stimulating the activity of hormone sensitive lipase and by inhibiting insulin action which in turn is caused both by decreased levels of insulin receptors and inhibition of insulin signaling. See Figure 7. GH enhances blood glucose by stimulating gluconeogenesis in the liver and increased fatty acids inhibit glucose uptake in the muscles.
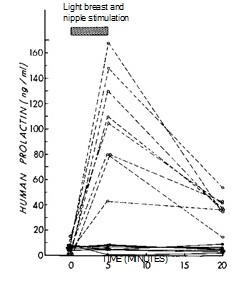
Prolactin promotes lactation in the breast. Prolactin, insulin, and cortisol are all required for lactation, and oxytocin is required to make the milk available to the baby. Prolactin regulates spermatogenesis in men through effects on GnRH and testosterone. Males and females both secrete prolactin, but nipple stimulation results in prolactin release only in females (Figure 8). Prolactin and placental lactogen also contribute to the insulin resistance that is normally associated with late pregnancy.
The glycoprotein hormones in the anterior pituitary include LH, FSH, and TSH. Chorionic gonadotropin (CG) is a placental hormone closely related to LH. These hormones are composed of two subunits with a combined molecular weight of 30 kDa. The α subunits are in common for each of these hormones, while the β subunits are different and are responsible for the distinct hormone activities.
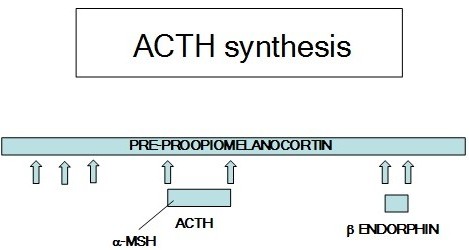
The corticotrophin-related proteins include adrenocorticotropic hormone (ACTH), melanocyte stimulating hormone (MSH), and certain endorphins and enkephalins.
These hormones are derived from a single precursor protein, preproopiomelanocortin (POMC)(Figure 9). ACTH stimulates the adrenal cortex to secrete glucocorticoids. The endorphins and enkephalins have opiate-like actions that reduce sensitivity to pain. alpha-MSH causes a sunlight independent skin tanning response of the skin.
Hypothalamic Releasing Hormones
Hormones are released from the hypothalamus into the portal circulation called the median eminence to regulate secretion of the anterior pituitary hormones. The identification of these releasing hormones required isolation from hundreds of thousands of sheep and pig hypothalami (Table 3).
| Table 3: Hypothalamic releasing hormones. | ||
| Releasing Hormone (number of amino acids) | Anterior Pituitary Hormones | Stimulate↑ or Inhibit↓ |
| TRH (3) | TSH PRL |
↑ ↑ |
| GnRH (10) | LH FSH |
↑ ↑ |
| SST (14) | GH TSH |
↓ ↓ |
| Dopamine (1) | PRL | ↓ |
| CRH (41) | ACTH α-MSH β-endorphin |
↑ ↑ ↑ |
| GHRH (40-44) | GH | ↑ |
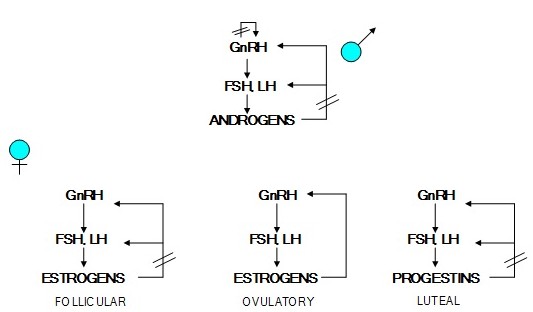
Patterns of Release
The hypothalamic releasing hormones and the anterior pituitary hormones they control are released in a pulsatile fashion. Figure 10 shows these relationships between GnRH and LH and estrogen. If GnRH is administered in a constant dosage, the pituitary cells that produce LH become less sensitive to GnRH because of a reduced number of GnRH receptors. Similarly, if LH is infused at a constant level, the steroid hormone-producing cells in the ovaries or testes become less responsive to LH because of a reduced number of LH receptors. Estrogen suppresses the release of GnRH and LH.
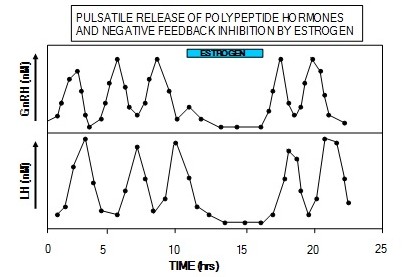
In addition to the pulsatile pattern of release, there is also a circadian rhythm to the secretion of these hormones such that most of the hormone is secreted late at night. In the case of GH and cortisol, the diurnal pattern helps maintain blood glucose during the overnight fast. This diurnal pattern also makes the normal range of these hormones quite large.
Feedback Control Mechanisms between the Hypothalamus, Anterior Pituitary, and Target Organs
Hormones secreted by the target organs of pituitary hormones often participate in the feedback regulation of their own trophic hormones. For example, administration of glucocorticoids suppresses ACTH and CRH and thereby decreases glucocorticoid production by the adrenal cortex (Figures 11 and 12). Similarly, in the follicular phase of the menstrual cycle, estrogen suppresses both LH and GnRH.
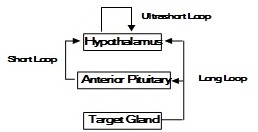
The exact patterns of negative feedback control are different for each hormone, but long loop, short loop, and ultrashort loop feedback pathways have been described in humans (Figure 11). Long loop feedback is like those described above for glucocorticoids and estrogen. The hormone produced by the target organ inhibits the release of the trophic hormone. This inhibition may be at the level of the pituitary, the hypothalamus, or both. Short loop feedback refers to the pituitary hormone directly feeding back to its releasing hormone in the hypothalamus. For example, GH negatively feeds back to inhibit GHRH release. IGF-1 functions in a long loop to negatively regulate GH release directly in the pituitary and indirectly by stimulating SST release from the hypothalamus. Grehlin, a gut hormone noted for stimulating hunger, synergizes with GHRH to enhance GH release. Ultrashort loop feedback refers to the ability of some hypothalamic releasing hormones to directly inhibit their own further release. Ultrashort loop feedback applies to the secretion of GnRH and SST (Figures 12 and 13).
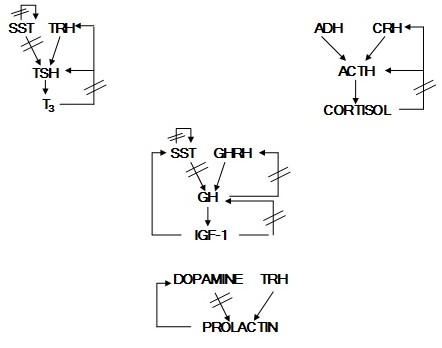
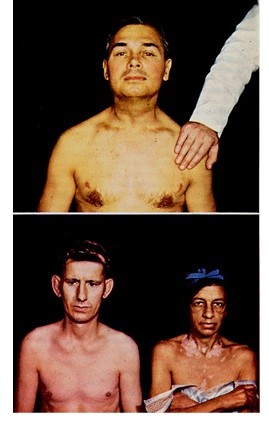
These feedback loops can be used to better understand endocrine disorders. For example, patients with hypoadrenalism (low glucocorticoids) may have high or low levels of ACTH. If the primary problem is in the adrenal cortex (Addison’s disease), glucocorticoids will be low and there will be less feedback inhibition on CRH and ACTH. The ACTH level may increase so much in these patients that the α-MSH sequence within the ACTH molecule can cause skin darkening in light-skinned patients(Figure 14). In dark skinned individuals, this can be noted in skin creases and the gums. ADH can also be elevated in these cases due either to CRH stimulating ADH release of lack of cortisol negative feedback on ADH release. On the other hand, if the patient’s primary problem is in the pituitary or hypothalamus, glucocorticoid levels will be low with ACTH levels also being low. These hypoadrenal patients will not be hyperpigmented.

Feedback/Errata